PureTech’s LYT-100 (Deupirfenidone) Demonstrates Tolerability and Pharmacokinetic Proof-of-Concept in Phase 1 Multiple Ascending Dose and Food Effect Study
LYT-100 was well-tolerated at all pre-specified doses, so an additional cohort of 1000 mg twice a day was added, which was also well-tolerated
In a previous study, a single dose of 801 mg of LYT-100 yielded greater exposure than a single dose of 801 mg (FDA-approved dose) of pirfenidone
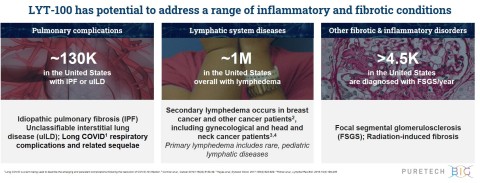
LYT-100 to be advanced for conditions involving inflammation and fibrosis and disorders of lymphatic flow, including idiopathic pulmonary fibrosis, Long COVID and lymphedema
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201117006303/en/
LYT-100 is a deuterated, oral small molecule designed to overcome the challenges associated with pirfenidone, an approved and marketed anti-inflammatory and anti-fibrotic drug. Pirfenidone is currently approved for the treatment of idiopathic pulmonary fibrosis (IPF), but it is associated with significant tolerability issues and dose-limiting toxicities leading approximately 50% of patients to discontinue use, dose adjust or switch therapies, which results in suboptimal disease management.1 LYT-100, a new chemical entity, retains the pharmacology of pirfenidone but has a differentiated PK profile, which is designed to enable improved tolerability, less frequent dosing and potentially increased efficacy.
“The strong results from this Phase 1 readout reinforce our view that LYT-100 has the potential to offer a tolerability and bioavailability profile that could be highly differentiated at the same exposure levels as pirfenidone,” said
Multiple ascending dose and food effect study results
The Phase 1 multiple ascending dose and food effect study was a randomized, double-blind study designed to evaluate the safety, tolerability and PK profile of LYT-100 in healthy participants. In the multiple ascending dose part of the study, participants were initially scheduled to receive increasing doses of LYT-100 up to 750 mg BID. LYT-100 was well-tolerated at all pre-specified doses, so an additional cohort receiving 1000 mg BID was assessed. LYT-100 was well-tolerated at that dose as well. In the food effect portion of the study, participants received a single dose of 500 mg of LYT-100 with or without food.
All adverse events (AEs) that were possibly or probably related to LYT-100 were mild and transient and there were no discontinuations. The most common AEs across all multiple ascending dose cohorts were headache (23.3% with LYT-100 vs. 20.0% with placebo), abdominal distension (10.0% with LYT-100 vs. 0% with placebo), nausea (10.0% with LYT-100 vs. 0% with placebo) and abdominal discomfort (6.7% with LYT-100 vs. 10.0% with placebo). The only AEs observed in the highest dose cohort (1000 mg BID) that were considered possibly related to LYT-100 were two headaches. No serious AEs or dose-limiting toxicities were observed in the study, and there was no dose response for any type of AE. The maximum tolerated dose was not observed during this study, suggesting higher doses could be explored.
The food effect portion of the study evaluated two common PK measures that are used to determine the dose of a product candidate – area under the curve (AUC), which represents exposure, and Cmax, which reflects the maximum concentration following drug administration. AUC and Cmax were each observed to decrease when LYT-100 was taken with food as compared to fasting conditions. Under fed conditions, the AUC reduction observed with LYT-100 (19%) was comparable to the AUC reduction stated in the ESBRIET® (pirfenidone) US Prescribing Information (16%). The Cmax reduction observed with LYT-100 was 23%, while the Cmax reduction stated in the ESBRIET® (pirfenidone) US Prescribing Information is 49%. Based on the food effect findings,
The therapeutic dose of pirfenidone approved by the
Based on these results,
About LYT-100
LYT-100 is PureTech’s most advanced wholly-owned product candidate. A deuterated form of pirfenidone, an approved anti-inflammatory and anti-fibrotic drug, LYT-100 is being advanced for the potential treatment of conditions involving inflammation and fibrosis and disorders of lymphatic flow, including lung dysfunction conditions (e.g., IPF, unclassifiable interstitial lung diseases (uILDs), Long COVID respiratory complications and related sequelae) and lymphedema.
About
For more information, visit www.puretechhealth.com or connect with us on Twitter @puretechh.
Forward Looking Statement
This press release contains statements that are or may be forward-looking statements, including statements that relate to the company's future prospects, developments, and strategies. The forward looking statements are based on current expectations and are subject to known and unknown risks and uncertainties that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, our expectations regarding the potential therapeutic benefits of LYT-100, our plans to initiate a Phase 2 trial evaluating LYT-100 as a potential treatment for Long COVID respiratory complications and related sequelae and a Phase 2a proof-of-concept study evaluating LYT-100 in patients with breast cancer-related, upper limb secondary lymphedema, our plans to advance LYT-100 for the treatment of IPF and those risks and uncertainties described in the risk factors included in the regulatory filings for
1 Cottin, V., Koschel, D., Günther, et al. (2018). Long-term safety of pirfenidone: Results of the prospective, observational PASSPORT study.
View source version on businesswire.com: https://www.businesswire.com/news/home/20201117006303/en/
Investors
+1 617 651 3156
amt@puretechhealth.com
EU media
+44 (0) 20 3727 1000
ben.atwell@FTIconsulting.com
US media
+1 617 581 9333
stephanie@tenbridgecommunications.com
Source:
